Performance Parameters:
1.Measurement Areas: Radius and Tibia.
2.Measurement Mode: Dual emission and dual receiving for enhanced accuracy.
3.Measurement Parameters: Speed of Sound (SOS).
4.Data Analysis Metrics: T-Score, Z-Score, Age Percentile [%], Adult Percentile [%], Bone Quality Index (BQI), Physiological Age of Bone (PAB [Year]), Expected Osteoporosis Age (EOA [Year]), Relative Fracture Risk (RRF).
5.Power Supply: External medical-grade power supply, power output >60W.
6.Connectivity: Internet connectivity with remote service capabilities.
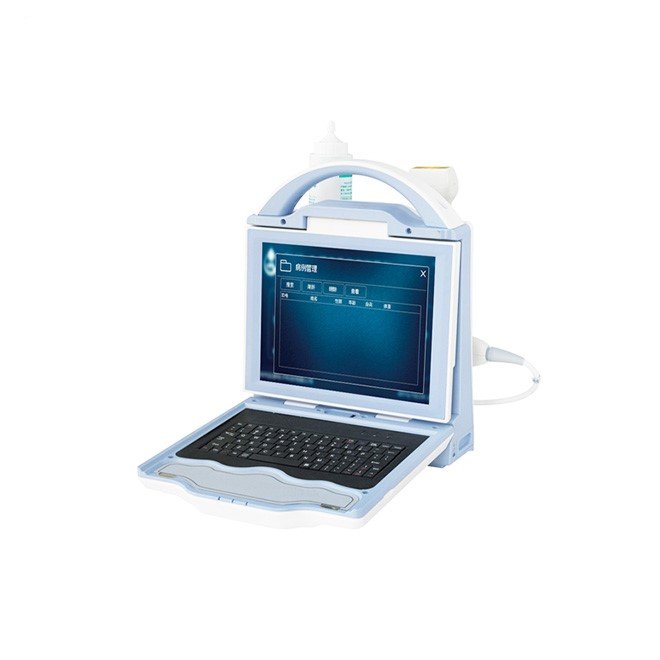
7.Display: 10.4” HD color LED monitor for clear visualization.
8.Fluid Protection: Main unit waterproof level IPX0, probe waterproof level IPX7.
9.Portability: Main unit weighs less than 4 kg, making it highly portable.
10.Printer: Optional printer available.
Technical Features:
1.Dry Technology: Fully dry diagnostic technology for increased convenience and reliability.
2.Advanced Probe Technology: Utilizes DuPont technology from the USA, offering superior sensitivity and reception.
3.Enhanced Frequency: Optimized ultrasonic frequency at measuring positions for better penetration and effective signal reception.
4.Ultrasonic Conduction Technology: Employs double emission and double receiving techniques for precise data collection.
5.Innovative Algorithms: Equipped with advanced algorithms to minimize interference and ensure valid data output.
6.System Correction: Features a specialized correction system to reduce system errors and enhance measurement accuracy.
7.High Shielding and Mold Manufacturing: Multi-point access mode ensures lossless ultrasonic signal transmission.
8.Comprehensive Clinical Database: Includes data sets for various races, including European, American, Asian, Chinese, and WHO standards, suitable for patients aged 0 to 100.
9.User Interface: English menu for easy navigation.
10.Safety Compliance: The first device in its class to pass the EMC test.